
Because your comment will be made public, you are solely responsible for ensuring that your comment does not include any confidential information that you or a third party may not wish to be posted, such as medical information, your or anyone else's Social Security number, or confidential business information, such as a manufacturing process. Comments submitted electronically, including attachments, to will be posted to the docket unchanged.

Follow the instructions for submitting comments. Submit electronic comments in the following way: Comments received by mail/hand delivery/courier (for written/paper submissions) will be considered timely if they are postmarked or the delivery service acceptance receipt is on or before that date. Eastern Time at the end of January 22, 2019. The electronic filing system will accept comments until 11:59 p.m. Electronic comments must be submitted on or before January 22, 2019. Please note that late, untimely filed comments will not be considered. Submit either electronic or written comments by January 22, 2019. This document is not intended to communicate FDA's proposed (or final) regulatory expectations but is instead meant to seek early input from groups and individuals outside the Agency prior to development of a draft guidance. The proposed framework is being issued for discussion purposes only and is not a draft guidance.
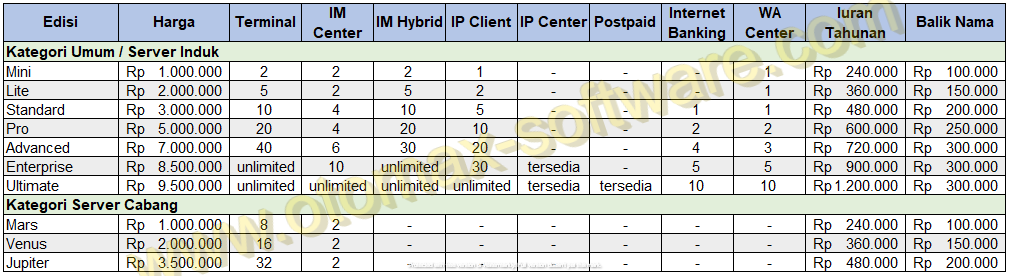
#OTOMAX SOFTWARE SOFTWARE#
Software that is developed for use with prescription drugs but is not disseminated by or on behalf of a drug sponsor is not addressed in this proposal. For purposes of the notice, prescription drug-use-related software refers to software disseminated by or on behalf of a drug sponsor that accompanies one or more of the sponsor's prescription drugs (including biological drug products). The framework proposed in this notice focuses not on prescription drug-use-related software itself, but rather on the output of such software that is presented to the end user. Recognizing the opportunities for increased use of digital technology with prescription drugs, the Agency is proposing a framework that would provide prescription drug sponsors the flexibility to develop and disseminate innovative software, while maintaining appropriate Agency oversight over the sponsors' communications about their products. The Food and Drug Administration (FDA or the Agency) is announcing the establishment of a docket to solicit public comment on a proposed framework for regulating software applications disseminated by or on behalf of drug sponsors for use Start Printed 75with one or more of their prescription drug products. Notice establishment of a public docket request for comments. Additional Issues for Considerationįood and Drug Administration, HHS. Output of Prescription-Drug-Use-Related Software That Has Been Cleared or Approved by CDRH Output of Prescription Drug-Use-Related Software That Contains Multiple Functions Prescription Drug-Use-Related Software Output That Constitutes Promotional Labeling Information About Prescription Drug-Use-Related Software Output That May Be Included in FDA-Required Labeling Prescription Drug-Use-Related Software Output as Labeling for a Prescription Drug FDA's Proposed Framework for Prescription Drug-Use-Related Software Output This repetition of headings to form internal navigation links Headings within the legal text of Federal Register documents. This table of contents is a navigational tool, processed from the Provide legal notice to the public or judicial notice to the courts. Rendition of the daily Federal Register on does not Until the ACFR grants it official status, the XML Legal research should verify their results against an official edition of
#OTOMAX SOFTWARE PDF#
The official SGML-based PDF version on, those relying on it for The material on is accurately displayed, consistent with While every effort has been made to ensure that Regulatory information on with the objective ofĮstablishing the XML-based Federal Register as an ACFR-sanctioned The OFR/GPO partnership is committed to presenting accurate and reliable Register (ACFR) issues a regulation granting it official legal status.įor complete information about, and access to, our official publications Informational resource until the Administrative Committee of the Federal This prototype edition of theĭaily Federal Register on will remain an unofficial

Each document posted on the site includes a link to theĬorresponding official PDF file on. The documents posted on this site are XML renditions of published Federal Register, and does not replace the official print version or the official It is not an official legal edition of the Federal This site displays a prototype of a “Web 2.0” version of the dailyįederal Register.


 0 kommentar(er)
0 kommentar(er)
